Abstract
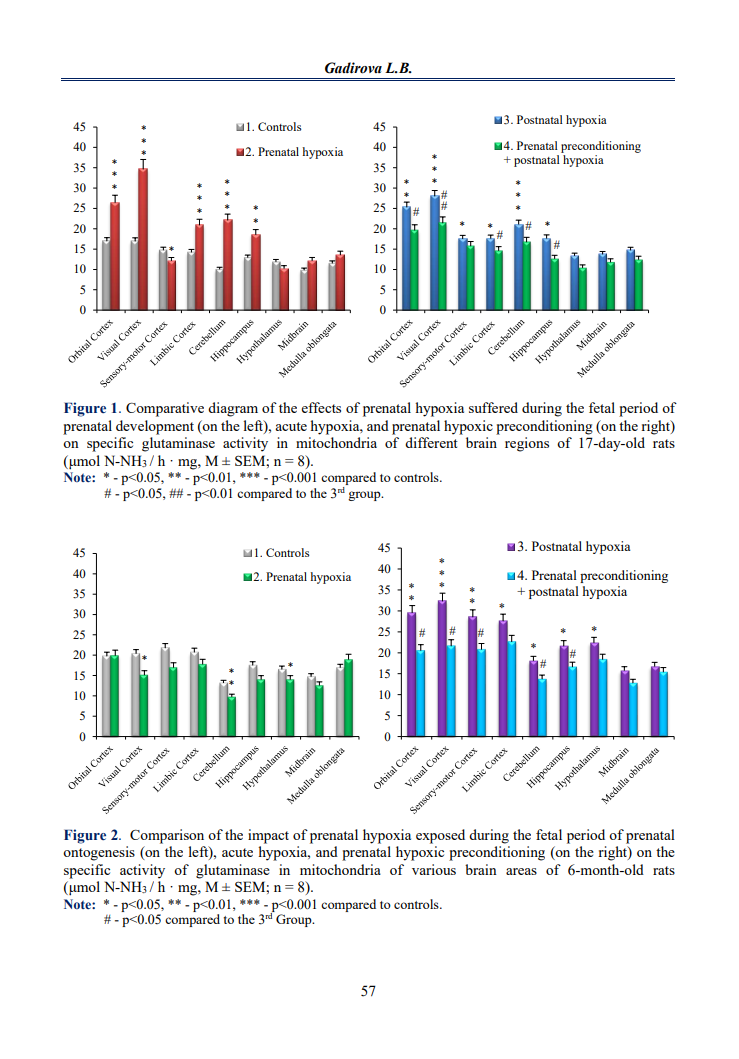
The hypoxic preconditioning model is widely used in experimental studies to identify mechanisms for increasing the tolerance of organisms to subsequent hypoxic exposure. Glutaminase is the main enzyme that synthesizes glutamate, which has two important physiological roles: in the postnatal period, it acts as an excitatory neurotransmitter, and during the prenatal period, it regulates neurogenesis, synaptogenesis, and the survival of nerve cells. In our work, in 4 experimental groups, we investigated the effect of hypoxic preconditioning performed during days 16–21 of pregnancy on glutamate synthesis in the brains of 17-day-old and 6-month-old offspring. It was found that prenatal hypoxia led to a pronounced increase in the enzyme activity in various brain structures in early postnatal ontogenesis, while a decrease was observed in adult animals. In contrast, exposure to acute hypoxia resulted in a more significant increase in glutaminase activity in the brains of adult animals. Prenatal fetal hypoxic preconditioning caused a weakening effect on the increase in enzyme activity in 17-day-old rat offspring and a down-regulation in 6-month-olds, compared to the group that suffered acute hypoxia in the postnatal period. Thus, a neuroprotective adaptive-compensatory effect of prenatal preconditioning has been demonstrated, which can be associated with both the physiological and excitotoxic effects of glutamate.
References
Chinopoulos C, Zhang SF, Thomas B, Ten V, Starkov AA. Isolation and functional assessment of mitochondria from small amounts of mouse brain tissue. Methods Mol. Biol. 2011;793:311-324.
Cox-Limpens KE, Vles JS, Schlechter J, Zimmermann LJ, Strackx E, Gavilanes AW. Fetal brain genomic reprogramming following asphyctic preconditioning. BMC Neurosci. 2013 Jun 22;14:61. https://doi.org/10.1186/1471-2202-14-61.
Dubrovskaya NM, Zhuravin IA. Ontogenetic characteristics of behavior in rats subjected to hypoxia on day 14 or day 18 of embryogenesis. Neuroscience and behavioral physiology. 2010;40(2):231–238. https://doi.org/10.1007/s11055-009-9235-2
Guseynov AG. The impact of hypoxic exposures in different periods of prenatal development on electrical activity of the rabbit auditory cortex in the first month of postnatal life. J Evol Biochem Phys. 2021 Nov;57:1277-89. https://doi.org/10.1134/S0022093021060089
[Гусейнов АГ. Влияние последствий гипоксических воздействий в разные периоды эмбриогенеза, на электрическую активность слуховой коры в первый месяц постнатального развития кроликов. Журнал эволюционной биохимии и физиологии. 2021:57(6):63–75.] https://doi.org/10.31857/S0044452921060048
Herlenius E, Lagercrantz H. Development of neurotransmitter systems during critical periods. Exp Neurol. 2004 Nov;190 Suppl 1:S8-21. https://doi.org/10.1016/j.expneurol.2004.03.027
Khairova VR. Changes of the activity of glutamine synthetase in an experimental model of hypoxic preconditioning. Azerbaijan Journal of Physiology. 2022;37(2):47-52. https://doi.org/10.59883/ajp.11.
Kruger, NJ. The Bradford Method for Protein Quantitation. In: Walker, J.M. (eds) The Protein Protocols Handbook. Springer Protocols Handbooks. Humana Press. 2002:15-21. https://doi.org/10.1385/1-59259-169-8:15
Magarlamov AG, Zaikin AA, Beliaeva LV. Direct phenol-hypochlorite method for determining glutaminase activity. Ukr Biokhim Zh (1978). 1979 Sep-Oct;51(5):549-51. Russian.
Maslov LN, Lishmanov YB, Emelianova TV, Prut DA, Kolar F, Portnichenko AG, Podoksenov YK, Khaliulin IG, Wang H, Pei J-M. Hypoxic preconditioning as novel approach to prophylaxis of ischemic and reperfusion damage of brain and heart. Angiology and Vascular Surgery. 2011;17(3):27-36.
[Маслов ЛН, Лишманов ЮБ, Емельянова ТВ, Прут ДА, Колар Ф, Портниченко АГ, Подоксёнов ЮК, Халиулин ИГ, Ванг Х, Пей Ж-М. Гипоксическое прекондиционирование, как новый подход к профилактике ишемических и реперфузионных повреждений головного мозга и сердца. Ангиология и сосудистая хирургия. 2011;17(3):27-36.]
Miyazaki, T., Yamasaki, M., Hashimoto, K., Kohda, K., Yuzaki, M., Shimamoto, K., Tanaka, K., Kano, M., & Watanabe, M. Glutamate transporter GLAST controls synaptic wrapping by Bergmann glia and ensures proper wiring of Purkinje cells. Proceedings of the National Academy of Sciences. 2017;114(28):7438-7443. https://doi.org/10.1073/pnas.1617330114
Rashidova AM. Effect of hypoxic preconditioning on lactate dehydrogenase activity in the brain of albino rats exposed to prenatal hypoxia. Azerbaijan Journal of Physiology. 2022;37(1):89-96. (in Azerbaijani).
Rəşidova AM. Hipoksik prekondisionlaşmanın prenatal hipoksiya olmuş ağ siçovulların baş beynində laktatdehidrogenazanın fəallığına təsir effekti. Azerbaijan Journal of Physiology. 2022;37(1):89-96. https://doi.org/10.59883/ajp.23.
Retz W, Kornhuber J, Riederer P. Neurotransmission and the ontogeny of human brain. J Neural Transm (Vienna). 1996;103(4):403-19. https://doi.org/10.1007/BF01276417.
Swann JW, Hablitz JJ. Cellular abnormalities and synaptic plasticity in seizure disorders of the immature nervous system. Ment Retard Dev Disabil Res Rev. 2000;6(4):258-67. https://doi.org/10.1002/1098-2779(2000)6:4<258::AID-MRDD5>3.0.CO;2-H.
Taie S, Ono J, Iwanaga Y, Tomita S, Asaga T, Chujo K, Ueki M. Hypoxia-inducible factor-1 alpha has a key role in hypoxic preconditioning. J Clin Neurosci. 2009;16(8):1056–1060. https://doi.org/10.1016/j.jocn.2008.09.024
Vasiliev, DS, Tumanova, NL, Zhuravin, IA. Structural changes in the neocortex nervous tissue in rat ontogenesis after hypoxia at various terms of embryogenesis. J Evol Biochem Phys. 2008;44:304–315. https://doi.org/10.1134/S002209300803006X
Vazquez-Valls E, Flores-Soto ME, Chaparro-Huerta V, Torres-Mendoza BM, Gudiño-Cabrera G, Rivera-Cervantes MC, Pallas M, Camins A, Armendáriz-Borunda J, Beas-Zarate C. HIF-1α expression in the hippocampus and peripheral macrophages after glutamate-induced excitotoxicity. J Neuroimmunol. 2011 Sep 15;238(1-2):12-8. https://doi.org/10.1016/j.jneuroim.2011.06.001.

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright (c) 2023 Azerbaijan Journal of Physiology